Instructions for authors
Update 08-03-2023
Acta Medica Peruana (AMP) is the official scientific publication of the Peruvian College of Physicians; its objective is to spread medical knowledge to the medical and scientific community both nationally and internationally. It publishes papers in Spanish or English, on a quarterly basis.
AMP only receives original contributions (related with all medical specialties, clinical, surgical, as well as those dealing with public health issues, basic sciences, and medical education). These contributions may be included within the following categories:
- Editorial
- Original papers
- Short original papers
- Review articles
- Case reports
- Special articles
- History of medicine
- Letters to the editor
Every manuscript received by AMP is primarily evaluated by the Editorial Committee; if deemed as of interest for publication, if complying with formal requirements of instructions for authors, as well as with ethical and methodological requisites, it will undergo peer review (experts in this particular field) before being considered for publication.
The Instructions for authors follow recommendations from the International Committee of Medical Journal Editors (ICMJE), the World Association of Medical Editors (WAME), the Committee on Publications Ethics (COPE), as well as with requisites stated by SciELO and MEDLINE.
Regarding the editorial area, there are the standards by the International Organization for Standardization – ISO (www. iso. org) and the International System of Measures or Units (https://www. bipm. org/en/home).
You can download the Instructions for Authors - PDF version at: https://amp.cmp.org.pe/index.php/AMP/libraryFiles/downloadPublic/10
CONSIDERATIONS OF AUTHORITY AND INSTITUTIONAL AFFILIATION
First page
It must include the following:
- Title in Spanish and English; preferably, with a 20-word maximum extension. Also, a 10-word or less secondary title may be included.
- Authors list, including the following information for each one of them:
- Name (as they may want it to appear in the publication) and data base.
- Affiliation (two as a maximum; one institutional and the other academic).
- Professional group and specialty, also, the highest academic degree obtained.
- Current e-mail.
- Phone number.
- ORCID number for each author.
- Authorship contributions. Contributions from each author for the manuscript must be clearly stated. These must comply with the four points stated by ICMJE and must be presented in detailed in the sworn affidavit.
- Financing source.
- Disclosure of potential conflicts of interest. Every relationship, condition, or circumstance that might affect objectiveness when interpreting the paper must be declared. This may be an economical or institutional potential conflict (consultancy, scholarships, travel grants, travel allowances, etc.). The response generated when using the COI format must be included.
- Acknowledgements (when appropriate, and stating the reason).
- Corresponding author, his/her address, telephone number, and e-mail.
Authorship and institutional affiliation(s)
The complete list of involved persons, their order of appearance, and their institutional affiliations are the sole responsibility of the signing authors. Every authorship conflict or any ethical issues related with this will be settled according to the COPE rules.
Recognition of who is the author is based on ICMJE recommendations; therefore, each author must comply with these four criteria, with no exceptions:
1) Significant contributions for conceiving or designing the manuscript, or contributions for data collection, analysis, or interpretation;
2) Every important writing contribution or critical review of the manuscript contents;
3) Final approval of the version to be published; and
4) Assume responsibility with respect to every aspect dealt with in the manuscript, aiming to guarantee that every issue related with accurateness or integrity of any section in the paper will be adequately looked for and solved.
Academic degrees or professional groups are not determinants for establishing authorship(s) in a manuscript. Only contributions for developing the manuscript according to established authorship criteria must be taken into account.
Fundraising, data collection, or general supervision of the research group per se does not justify authorship. This information should be included in the acknowledgement section. Every author must detail what were his/her specific contributions for the manuscript and this information will be included in the paper, due to transparency reasons.
With respect to the order of appearance for the authors; in general, the first author is the one that worked the most and who wrote the first draft of the manuscript, and the last author usually is the junior researcher in the team. Every paper must state a single corresponding author. There may be exceptional cases when two corresponding authors may be included. These individuals are the ones that will be contacted by AMP with respect to the editorial process.
Those persons that may be included within the author list not complying with authorship criteria are called honorary authors. The aforementioned inclusion is considered as an ethical misconduct. Persons excluded from the author list are considered as ghost authors and this situation also is considered as an ethical misconduct. In case any person should consider he/she has been excluded from authorship, he/she may send a communication stating the evidence that he/she complied with authorship criteria. Should AMP detect this misconduct, COPE rules will be applied.
Every author must include his/her institutional affiliation(s), which must correspond to the institution where he/she is currently working or studying, and that may have contributed in some way for the development of research to be published. Up to two affiliations per author may be included. The ‘independent author’ term is also accepted.
The corresponding author must send the following data: address, telephone number, and e-mail. Any communication with respect to manuscript review and edition will be sent to this author.
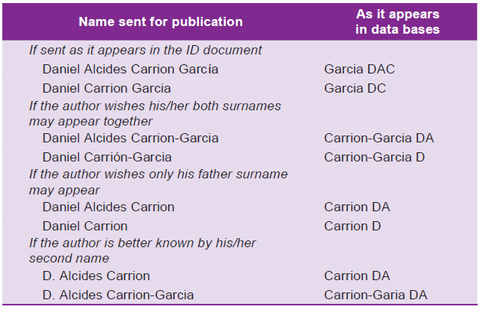
Authors must show the way in which they wish their name(s) should appear in the publication and in the data bases. They should take into account that data bases are written in English and that they only consider the last name as surname. Here we show some ways for presenting names and how they would appear in data bases:
Table 1. Examples for coding authors’ names in data bases.
Summary and keywords
Every article, except Editorials and Letters to the Editor, must have a summary in Spanish and in English. Also, key words in Spanish must be included, using Health Sciences Descriptors (DeCS/MeSH) (https://decs.bvsalud.org/en/current-decs/), two words as a minimum and six words as maximum.
.
References
Only those references cited within the text will be included, as a single reference [1], or if more than one reference is included [5–8], following a correlative order and between brackets. Vancouver format must be used, according to the ICMJE (https://www.nlm.nih.gov/bsd/unbiform_requirements.html). In case there are more than six authors, the first six will be included followed by ‘et al’. Also, the reference title may include a link directed towards the website where the reference may be accessed to, as long as the electronic version is available. Every reference must include its DOI number, when corresponding. Here we present some examples for references:
Published papers with DOI
Marmot M. Universal health coverage and social determinants of health. Lancet. 2013; 382 (9900): 1227-8. doi: 10.1016/S0140-6736(13)61791-2.
Sanchez Clemente N, Ugarte-Gil CA, Solorzano N, Maguiña C, Pachas P, Blazes D, et al. Bartonella bacilliformis: a systematic review of the literature to guide the research agenda for elimination. PLoS Negl Trop Dis. 2012; 6 (10): e1819. doi: 10.1371/journal.pntd.0001819.
Internet articles without DOI
Abood S. Quality improvement initiative in nursing homes: the ANA acts in an advisory role. Am J Nurs [Internet]. 2012 [citado el 12 de agosto de 2019]; 102(6):23-29. Disponible en: https://insights.ovid.com/article/00000446-200206000-00031.
Printed articles without DOI
Rose ME, Huerbin MB, Melick J, Marion DW, Palmer AM, Schiding JK, et al. Regulation of interstitial excitatory amino acid concentrations after cortical contusion injury. Brain Res. 2002;935(1-2):40-6.
Books
Hulley SB, Cummings SR, Browner WS, Grady DG, Newman TB. Designing clinical research. 4th ed. Philadelphia: Lippincott, Williams & Wilkins; 2013.
Chapters in books
McMillan W. Theory en healthcare education research: the importance of worldview. In: Cleland J, Durning SJ (editors). Researching medical education. Oxford: Willey Blackwell; 2015. p. 25-34.
Book on the internet
Manso G, Hidalgo A, Carvajal A, de Abajo FJ. Los primeros 25 años del sistema español de farmacovigilancia de medicamentos de uso humano [Internet]. Principado de Asturias: Universidad de Oviedo; 2010 [citado el 20 de febrero de 2017]. Disponible en: https://www.unioviedo.es/gaife/documentos/libro25aniversario/libro.pdf.
Chapters of books, pamphlets or similar
Fuente C, Rodríguez A, de Abajo FJ, Vargas E, Moreno A. Comité de Seguridad de Medicamentos de Uso Humano y su contribución a la salud pública. En: Manso G, Hidalgo A, Carvajal A, de Abajo FJ. Los primeros 25 años del sistema español de Farmacovigilancia de medicamentos de uso humano [Internet]. Principado de Asturias: Universidad de Oviedo; 2010 [citado el 14 de octubre de 2017]. p. 157-71. Disponible en: https://www.unioviedo.es/gaife/documentos/libro25aniversario/libro.pdf
Knowles NJ, Hovi T, Hyypiä T, King AM, Lindberg AM, Pallansch MA, et al. Picornaviridae. In: King AM, Adams MJ, Carstens EB, Lefkowitz EJ, editors. Virus taxonomy: classification and nomenclature of viruses: Ninth Report of the International Committee on Taxonomy of Viruses. San Diego: Elsevier; 2012. Pp. 855–80.
Thesis
Pesce H. La epidemiología de la lepra en el Perú [Tesis Doctoral]. Lima: Facultad de Medicina, Universidad Nacional Mayor de San Marcos; 1961.
Website
International Committee of Medical Journal Editor [website]. Defining the role of authors and contributors. Vancouver: ICMJE; 2015 [accessed on February 1st, 2016]. Available at: http://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html.
Tables
These must be presented after the references, each in a different page. Tables must be orders using Arabic numbers and they must include all necessary information, both contents and title, so they may be interpreted without the need for going back to the text. Only a single horizontal line separating the table title from its body will be accepted. In no case vertical lines shall be accepted. Tables must be written using Word or Excel, no image formats (.jpg, .png, etc.) will be accepted, since tables must be presented in a format that is amenable for diagramming. All abbreviations and symbols used in the table must include their explanation(s) on their inferior part.
Figures
Statistics graphs, flow charts, diagrams, photographs, maps, or correlatively ordered numeric charts may be included as figures, and they may be placed after tables. Statistics graphs and flow charts may be presented in Excel or in currently used statistical packages. Other images may be presented using TIFF or JPG formats, with >600- dpi or >300- pixel resolution, and they should be added in different archives, so that appropriate edition may be made, aiming to obtain a proper layout. Legends for micrographs must state power and staining method(s). Maps must have a scale.
Should any figure show patients’ faces, a dark area must be placed covering their eyes so they may not be identified; also, authors must attach written authorization from patients or their legal representatives allowing their consent in case of publishing photographs that might allow their identification. If a previously published figure is included, the origin source must be stated and a written permission from the copyright holder must be sent to AMP.
Considerations for style
AMP uses the International System of Units. Scientific names of species must be written using italics. Colons (,) are used for decimals in Spanish and dots (.) in English. Titles of papers must not have abbreviations. Should abbreviations be used in the text, the complete name must be placed and then the abbreviation between brackets after its first mention. The use of abbreviations is not recommended for items appearing less than four times in the paper. For stating percentages, a single decimal (i.e. 10.1%) is recommended. When mentioning populations less than 50 individuals, the use of percentages is not recommended, only fractions should be used (i.e. 20/50). When using association measurements such as odds ratios (OR) and their confidence intervals (CI), the use of two decimals is recommended (i.e. OR; 2, 15; 95% CI: 1,10–3, 41). For p-values, up to three decimals may be used (i.e. p= 0,009).
Section specific policies
AMP publishes different types of papers according to its editorial policy. The following table shows the maximum word length and other characteristics for the published papers:
Tabla 2. Maximum length admitted for publication, according to section(s).
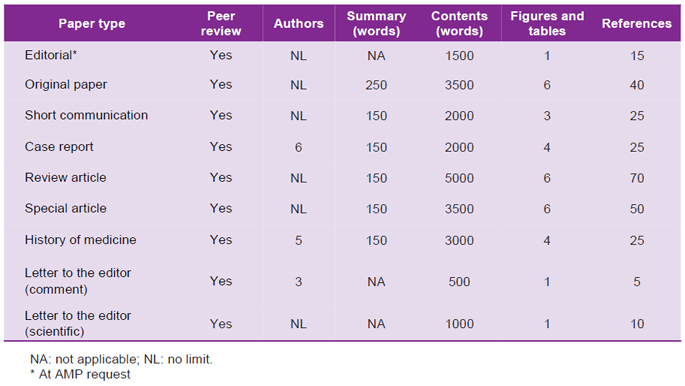
SECTIONS
AMP adheres itself to the Equator-Network initiative (https://www.equator-network.org), and recommends all authors to follow the guidelines adjusting to their study design. From 2021 on, the presentation of the check list included in this initiative will be mandatory, according to the paper type.
Editorial
This is presented under express request from AMP Editorial Committee; its contents must refer to a specific topic of interest about clinical practice, public health matters, issues related to medical activities or ethics, or AMP management or editorial policy. Editorials must have a specific title.
Original papers
These are products of original scientific research, and they must be related to a topic of interest for AMP. These are mainly studies using a prospective and analytical design, with a sample size that is adequate for the research question.
- Abstract. This is structured including objective(s), materials and methods, results, and conclusions. It must be written in Spanish and English.
- Keywords. The keywords in Spanish must be used using the Descriptors in Health Sciences (DeCS/MeSH) (https://decs.bvsalud.org/en/current-decs/), with a minimum of two and a maximum of six words.
- Introduction. This must be brief, going from general to specific features; it usually encompasses less than 20% of the total length of the paper. It must include what is known (relevant background), what is not known (related with the question of research), and what it is to be done (objectives).
- Materials and Methods. Here the used methodology is described, so that it would allow the study to be reproduced and data quality may be evaluated. It is recommended to review international consensus for presenting papers, according to the study design:
- Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) for writing observational studies (https://www.strobe-statement.org/).
- Standards for the Reporting of Diagnostic Accuracy Studies (STARD) for studies of diagnostic tests (http ://www.stard-statement.org/).
- Declaración Consolidated Standars of Reporting Trials (CONSORT) for clinical trials (http://www.consort-statement.org/).
- PRISMA for systematic reviews http://www.prisma-statement.org/
- SRQR for qualitative studies https://apps.who.int/iris/handle/10665/61113
- The Consolidated Health Economic Evaluation Reporting Standards (CHEERS) for economic evaluations https://www.sciencedirect.com/science/article/pii/S2212109913001313
- The CARE Guidelines: Consensus-based Clinical Case Reporting Guideline Development, for case reports: http://www.care-statement.org
These guides and useful information for the presentation of your articles can be found at EQUATOR
http://www.espanol.equator-network.org
The use of subtitles is recommended, including the following information as appropriate: - Study design. Here the study design is presented, alongside its dates and the place where it has been performed; describing the relevant issues that may help the reader for understanding the conditions in which the study had taken place and that might be useful in the discussion section.
- Study population. Here the study population is described, together with the selection criteria, sample size calculations and power as appropriate, sampling design and enrolling characteristics. It shows a flow chart describing how participants had been enrolled.
- Variables. Here the main study variables are described (relevant dependent and independent variables), so that the variable to be assessed has been assessed in the most appropriate manner, indicating the validity of the used method with its corresponding references, and also the cutoff points may be included in case categorical variables are used.
- Procedures or interventions. These are precisely described, so that any posterior replication of the study may be feasible. Medicinal plant collection and identification procedures are described when they are used. Pharmaceutical products and chemical compounds are to be properly identified, mentioning their active ingredient(s), generic name(s), dose, and administration route(s).
- Data analysis. Here how data is treated is described, including quality control for data bases, statistical software, p-values deemed as significant, tests used for variable assessment, data on assumption compliance and the ways in which models for multiple variables had been developed in case of use.
- Ethical considerations. This describes the approval by Ethics Committees, obtained authorizations, informed consent/assent, data confidentiality, and result return when appropriate.
- Results. There must be clearly presented, not including opinions or interpretations; unless they should correspond to statistical issues. Tables and texts must have a mention within the text, without repeating the information. These may include subtitles in order to facilitate their interpretation.
- Discussion. Here the main results are presented, which may respond to the study objectives. Results are compared with those from other trials, differences or similarities are presented, and reasons for this are stated. Limitations (bias) may be presented in this section, explaining why such findings do not invalidate the results obtained. Here, clinical implications of all findings are presented in detail, stating their importance for research or public health, and recommendations may be issued. Finally, conclusions summarizing the discussion may be presented, based on obtained results and responding to the study objectives.
Original article (Systematic reviews)
a. When the original article is a systematic review, we recommend that authors to review the PRISMA 2020 statement in the early writing process, because considering items prospectively can help ensure that all items are considered.
b. To assist in the follow-up of the items that have been reported, the PRISMA Declaration website (http://www. prisma-statement. org/) includes checklists templates that can be downloaded and completed (also available as Table 1 and Table 2 of the additional material).
c. There is also a web application that allows users to complete the checklist through a user-friendly interface (available at https://prisma. shinyapps. io/checklist/).
d. The complete list can be exported to Word or PDF format. In addition, editable flowchart templates can be downloaded from the PRISMA Statement website.
Short communications
These are also investigation products; and, due to their objectives, design, or results, they may be published using a format not exceeding 2000 words (see Table 2). These are particularly case series, non-probabilistic descriptive studies or small retrospective studies. They follow the same structure as that of an original article. The summary differs from that of an original article because it must not exceed 150 words, and it is not structured.
Review articles
These products should include a wide literature review, they should belong to the areas of interest of AMP, and they must follow this structure: have a non-structured 150-word summary, keywords, introduction, contents (structured according to the author’s discretion), discussion (including conclusions), and references.
Case reports
These products may include from one to ten cases describing an unusual condition, an atypical presentation of a common condition, unknown adverse events, rare disease associations, cases described for the first time in Peru, new interventions or new uses for drugs. All of these situations must have a clear message or lesson for the medical community. The use of CARE guide is recommended for preparing case reports (www.equator-network.org). These products have the following structure: non-structured summary, keywords, introduction (which generally describes the known facts), case report, discussion (where the learned lessons or contributions from presented cases are featured), and references. Information that may help in identifying patient(s) must not be included. In case photographs where it is unavoidable to show the patient’s face because of his/her condition, an explicit authorization from him/her or his/her legal representative must be included. Figures and photographs must be high quality, and they should be independently presented using .jpg or .tiff archives. A physician must always be the corresponding author, and treating physicians should be amongst other authors.
Special articles
These may be essays, opinion papers, clinical practice guides, systematization papers, research protocols, or experience reports that might be of interest for clinical practice, medical education, healthcare policy or being related to medical professional activity. These articles have the following structure: a not more than 150-word non-structured summary, keywords, introduction, contents (structured according to the author’s discretion), discussion (including conclusions), and references.
History of Medicine
Topics for historical review that are relevant in medical fields will be included. These may also include notes about deceased Peruvian physicians. These pieces do not have a summary, their structure is according to the author’s discretion, and the whole piece may include up to 2000 words.
Letters to the Editor
Scientific Letters. These pieces show systematically obtained results. Generally these are descriptive investigations using small non-probabilistic samples. These pieces may include case series or case reports showing specific results of interest or requiring fast-track publication. Scientific letters can have up to 1000 words and they are presented without a summary. These may also constitute a response to a previously published article in a former issue of AMP.
Comment Letters. In the case of non-scientific letters (describing investigation results), their extension may be up to 5000 words, five references one figure or table, and up to three authors. These letters may be a response to an article published in a former issue of AMP, therefore, extending the peer review process, and they may also correspond to evidence-based opinions about areas related to medical education, healthcare policy, being related to medical professional activity, or complaints related to ethical misconducts with respect to some article published in AMP. Implicated authors have the right to respond in the same or in the following issue. In exceptional cases there might be a rejoinder.
ETHICAL REGULATIONS FOR PUBLICATION
Acta Medica Peruana (AMP) follows ethical standards for scientific research and publication; therefore, when research has been carried out in humans, it is necessary to mention that the study has been approved by an Institutional Ethics Committee. The Editorial Committee reserves its right to request documented proof for this approval.
The following manuscripts do not require an approval by an Institutional Ethics Committee: 1) Studies using public domain secondary databases, including systematic reviews, meta-analyses, and bibliometric studies; 2) Public health surveillance interventions; 3) Research on outbreaks or sanitary emergency events; 4) Public health program evaluations; and 5) Educational evaluations scheduled within academic curricula.
The following manuscripts do require an approval by an Institutional Ethics Committee: 1) Research performed in human beings; 2) Research directly using human biological material or data from potentially identifiable human beings, such as data from biobanks or medical records. This case will apply as long as the previously presented exceptions may not be applicable.
The Editorial Committee suggests following the recommendations from these regulations and those from national and international instances: The World Medical Association Declaration of Helsinki (WMA, 2013), the WMA declaration on Ethical Considerations for Healthcare and Biobanks Data Bases (2016), the International Ethical Regulations for Health Related Research in Humans issued by the Council for International Organizations of Medical Sciences (CIOMS) in cooperation with the World Health Organization (WHO) (2017) and the Peruvian Regulations for Clinical Trials (Supreme Decree N° 021-2017-SA and its updated versions).
Ethics for publication
Acta Medica Peruana (AMP) adheres to the recommendations issued by the Committee in Publication Ethics (COPE; https://publicationethics.org) and the International Committee of Medical Journal Editors (ICMJE). AMP actively looks for potential faults against publication ethics, such as plagiarism, redundant publication, data manipulation and invention, as well as those related with authorship and institutional affiliation(s). Should such situations occur during the publication process, the manuscript will be rejected; in case the paper has been published, a rectification will be issued. This will take place after a proper investigation of the case has been performed and after the implicated author(s) had exerted their proper defense, following COPE flow charts. In both cases, competent institutions will be informed, i.e. the institutions where the author(s) work, their financing parties, their corresponding professional organizations and ethical committees that had approved the study, when appropriate. Also, the Ethics and Deontology Committee of the Peruvian College of Physicians will be informed, if deemed necessary.
In case of doubt about any ethical misconduct, author(s) may contact the Editorial Committee in order to obtain adequate orientation (actamedicaperuana@cmp.org.pe).
Potential conflicts of interests of members of the Editorial Committee
Any manuscript including any member of AMP Editorial Committee is not exempt from complying with the requisites specified in these instructions, and manuscripts to be processed will follow the same pathway that has been outlined for any paper; nonetheless, the process will take place without the participation of the involved Editorial Committee member. This means he/she will not be able to participate in any session regarding that particular manuscript (decision, review), and he will not know the names of the assigned reviewers. Also, members of the Editorial Committee will have to declare their potential conflicts of interest with respect to that particular manuscript, and they will inhibit themselves from decision making on that issue.
MANUSCRIPTS EDITORIAL PROCESS
Initial evaluation
Articles submitted to the OJS-AMP will be presented and posted to consideration of the Editorial Committee, which is made up of health professionals, members of leading institutions in investigation. The Editorial Committee will decide if the article is accepted to enter the editorial process when 1) corresponds to your line editorial and 2) is within one of the sections it publishes; later and 3) it has the characteristics of being novel, it has methodology consistent with the problem investigated and is written adequately; and will move it to the peer review process. In case Otherwise, the submission will not be accepted and returned to the author.
Any manuscript that includes a member of the AMP Committee as the author is not exempt from complying with the requirements specified in the instructions and its editorial process will be the same as any other Shipping; However, the latter will be carried out without the participation of the involved member; that is, you will not be able to participate in any session that deal with the manuscript (decision, revision) and you won't even know the name of assigned reviewers. In addition, the members of the Editorial Committee must declare potential conflicts of interest regarding said article, inhibiting himself from making decisions.
Peer review
Peer review is intended to ensure the quality of the articles to publish. This review is double blind. Selection of the reviewers are based on their expertise on the subject (proven through their publications and academic degree), or by their expertise in methodological issues (e.g. specialists in Biostatistics, Epidemiology, etc.). The review is ad honorem. Every reviewer has the obligation to declare possible conflicts of interest regarding the item ordered and inhibited from review; In addition, you must save the privacy of the data contained in the article.
The qualification can be concluded in: a) accepted without modifications; b) publishable with minor observations; c) publishable with comments greater; d) not publishable. In addition, the reviewer may recommend the publication of an article, but in another section of the journal (e.g. before original article as short original).
Based on the comments of the reviewers, the Editorial Committee finally decide if the article can be published, its not approval or sending comments to the author.
Response to comments
The author must send the raising of observations including: 1) the article corrected with exchange control and 2) a letter detailing each of the observations made, the response to these and the actions performed by the author. The inappropriate response of the Observations could be criteria for requesting clarification additional and even the non-approval of the article. AMP will be able to return to send the corrected article to a reviewer before considering its post.
The average period of the editorial process (from receipt to final decision of the Editorial Committee) may vary from two to four months, depending on the review process and the authors' response.
Printing tests
Once the edition is finished, the article will be sent to layout. Subsequently, the diagrammed article will be sent to the authors with the so that modifications, reductions or extensions can be made of the text or of the tables and figures, and finally give their approval of publication. When the authors do not respond within the term established by AMP for this step, the final version will be accepted.
English publication
The magazine allows the dissemination of articles accepted for publication (final version) in English. For this, the author must submit the accepted article for publication in English within the two week period.
OPEN ACCESS POLICY
This journal provides immediate open access to its content, based on the principle that to offer the public with free access to Research helps a greater global exchange of knowledge. The magazine uses the license Creative Commons Attribution 4.0 international .
ACCEPTANCE OF ORIGINALITY
Scientific articles, brief communications, case reports, special articles and/or other articles must be free of arbitration in another journal or, if applicable, be translations previously published in their original language (if it were the case). In addition, it is necessary that the texts sent are original and that they have not been partially published or that they are in the process of being published in another journal or dissemination medium. In any of its types, the articles immediately received will be evaluated for their academic honesty using the Turnitin software.
If the works that exceed 20% similarity and coincidence with another text, book, article, etc., they will be returned to their authors. If in the process the article has been accepted by another journal, the author must inform such condition. If so, the article will be withdrawn from the evaluation or editing process. It should be noted that the Editorial Committee, if it deems it necessary, has the power to request a declaration of originality of the articles.
The manuscripts received must be unpublished and original, whose themes revolve around health sciences in all its branches and related disciplines.
PAYMENTS PER RECEPTION OR PUBLICATION
Acta Médica Peruana, does NOT apply article processing charges Article processing charge (APC), in any of the accepted formats, which includes reception, preliminary review by the Editorial Committee, double-blind peer review, style correction, layout, publication, DOI assignment and article marking.
CONTACT
If you have any questions, you can contact us by email actamedicaperuana@cmp.org.pe or call: (+511) 213-1400, annex: 2602.








1.png)

.png)




